Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yanagida, Akinobu*; Ura, Yoko*; Mitsui, Seiichiro; Ishidera, Takamitsu; Kawakita, Ryohei
Nara Bunkazai Kenkyujo Soritsu 70-Shunen Kinen Rombunshu; Bunkazai Ronso 5, p.843 - 856, 2023/03
To investigate chloride salt accumulation inside an iron artifact in soil, non-destructive analysis of three iron artifacts excavated from the Heijo Palace Site was conducted using elemental mapping by X-ray fluorescence analysis, micro-X-ray diffraction analysis, and X-ray computed tomography. Furthermore, the buried environments of the artifacts were presumed based on the previous reports of the environmental investigation at the Heijo Palace site. The results revealed the iron artifact's corrosion behavior was different individually- (1) the iron artifact that was presumed buried under oxidation environments had a goethite/magnetite corrosion layer and contained akageneite inside the corrosion layer. (2) the metal of the other iron artifacts buried under the oxidation environment had eluted absolutely and the artifacts had a rust layer formed by only goethite. (3) the other artifact buried in reduction environments had a rust layer composed of siderite. Accumulation of chloride salts inside an iron artifact was observed only in (1). Because each Cl concentration measured in underground water observation holes at the Heijo Palace Site showed almost the same level concentrations, it was presumed that the accumulation of chloride salts depended on the environmental factor except for Cl
concentration measured in underground water observation holes at the Heijo Palace Site showed almost the same level concentrations, it was presumed that the accumulation of chloride salts depended on the environmental factor except for Cl concentration. Based on these results, there was a possibility that the occurrence of local corrosion attributed to the separation of anodic and cathodic regions through the formation of the goethite/magnetite rust layer caused chloride salts accumulation inside an iron artifact.
concentration. Based on these results, there was a possibility that the occurrence of local corrosion attributed to the separation of anodic and cathodic regions through the formation of the goethite/magnetite rust layer caused chloride salts accumulation inside an iron artifact.
Mitsui, Seiichiro
Koeki Zaidan Hojin Tottoriken Kyoiku Bunka Zaidanhen 2014 "Yoshida Nakamichi Iseki" Tottoriken Kyoiku Iinkai, p.221 - 230, 2015/03
An ancient socketed iron axe was excavated from Yoshida Nakamichi site in Tottori City, Tottori Prefecture. To understand reasons of corrosion state of the axe, we studied relationship between burial environment and corrosion. As environmental conditions, we investigated groundwater chemistry and corrosion rate with iron probe monitor, etc. As for corrosion state, we analysed corrosion depths with a X-ray CT and corrosion products with a portable XRD/XRF. As results, we found that the redox potential and dissolved oxygen level as environmental conditions were very low, and that the maximum corrosion rate (2 10
10 mm/y) evaluated from measured corrosion depths was smaller than the probe corrosion rate (5
mm/y) evaluated from measured corrosion depths was smaller than the probe corrosion rate (5 10
10 mm/y) by two orders of magnitude and identified siderite (FeCO
mm/y) by two orders of magnitude and identified siderite (FeCO ) as a corrosion product. The results suggested that the siderite precipitated on the surface of the iron sword inhibited corrosion reaction.
) as a corrosion product. The results suggested that the siderite precipitated on the surface of the iron sword inhibited corrosion reaction.
*
PNC TJ1211 95-007, 117 Pages, 1995/02
1. Survey on leaching rate of pyrite, chlorite, epidote and siderite. (1) Pyrite Reaction rate (K) is depended on dissolve O concentration in Lin's literature. 1 - (1-x)
concentration in Lin's literature. 1 - (1-x) 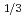 = k [O
= k [O ]
] 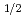 ・t (x: Mole number of dissolved FeS
・t (x: Mole number of dissolved FeS , [O
, [O ]: Dissolve O
]: Dissolve O concentration. T:Time) Reaction rate is changed by temperature. k = 2.2
concentration. T:Time) Reaction rate is changed by temperature. k = 2.2  10
10 exp(-9140/T) (2) chlorite Reaction rate is measured 2.7
exp(-9140/T) (2) chlorite Reaction rate is measured 2.7  6.7
6.7  10
10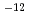 mol/m
mol/m /s(pH3
/s(pH3  4.5) by Swoboda-Colberg et al. Reaction rate is controlled by inner diffusion. (3) Epidote Reaction rate is measured 10
4.5) by Swoboda-Colberg et al. Reaction rate is controlled by inner diffusion. (3) Epidote Reaction rate is measured 10
 10
10 mol/cm
mol/cm /s in pH1
/s in pH1  11 by Rose. (4) Siderite Reaction rate is measured 9.93
11 by Rose. (4) Siderite Reaction rate is measured 9.93  10
10 mol/m
mol/m /s in O
/s in O -free solution by Greenberg et al. 2. Experimental study on leaching rate of minerals. (1) Measurement of leaching rate of minerals. Leaching rate of pyrite was measured in distilled waster under redox condition. Reaction rate (K) was estimated 10
-free solution by Greenberg et al. 2. Experimental study on leaching rate of minerals. (1) Measurement of leaching rate of minerals. Leaching rate of pyrite was measured in distilled waster under redox condition. Reaction rate (K) was estimated 10
 10
10 (cm
(cm ・mol
・mol ) 1/2・h
) 1/2・h by Lin's rate equation. At 56 days in the experiments. Alterated layer could not be found in SEM and EDX observation. (2) Measurement of leaching rate of chlorite Leaching rate of chlorite was measured in distilled waster under air condition. Disolution process of chloric was parabolic Stage, and reaction rate (K) was estimated order of 10
by Lin's rate equation. At 56 days in the experiments. Alterated layer could not be found in SEM and EDX observation. (2) Measurement of leaching rate of chlorite Leaching rate of chlorite was measured in distilled waster under air condition. Disolution process of chloric was parabolic Stage, and reaction rate (K) was estimated order of 10
 10
10 by Ross's rate equation.
by Ross's rate equation. 
 = kt [
= kt [  : disoleved mineral amount / intial mineral amount, t : time(sec) ] At 56 days in the experiments, alterated layer could not be found in SEM and EDX observation.
: disoleved mineral amount / intial mineral amount, t : time(sec) ] At 56 days in the experiments, alterated layer could not be found in SEM and EDX observation.